From OMOP to DDD
ARC I&A June 2025
2025-06-25
Terms
OMOP

The Observational Medical Outcomes Partnership is a public-private partnership that aims to improve the quality of observational research. OMOP provides a standardized data model and common data elements to facilitate the sharing and analysis of healthcare data across different organizations.
OMOP
OMOP’s Common Data Model (CDM) is an open community data standard, designed to standardize the structure and content of observational data and to enable efficient analyses that can produce reliable evidence.
OMOP
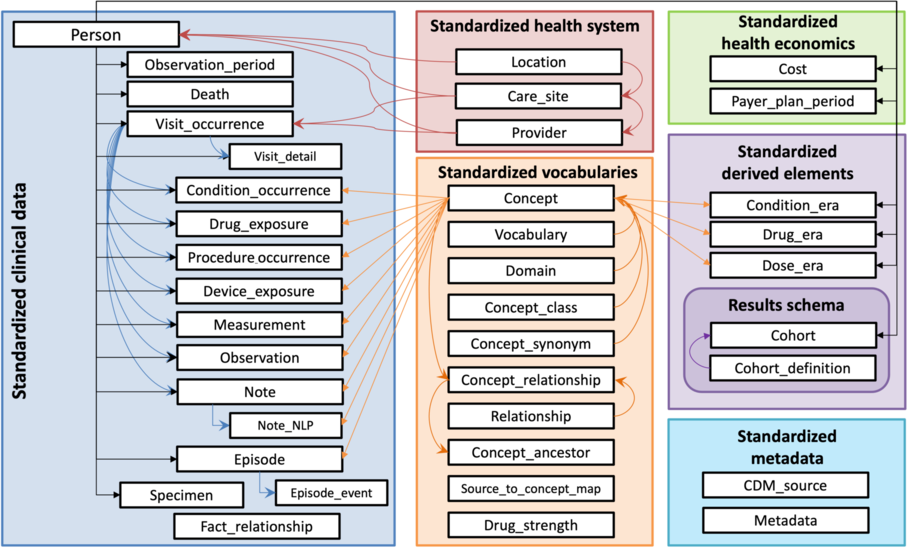
Common Data Model
DDD
Defined Daily Dose is a unit of measurement used to quantify the amount of a drug that is typically prescribed for a specific condition. It is often used in pharmacology and healthcare research to standardize the measurement of drug consumption.
DDD
It is defined by the World Health Organization (WHO) and is based on the average maintenance dose of a drug for its main indication in adults. It allows for comparisons of drug usage across different populations and settings.
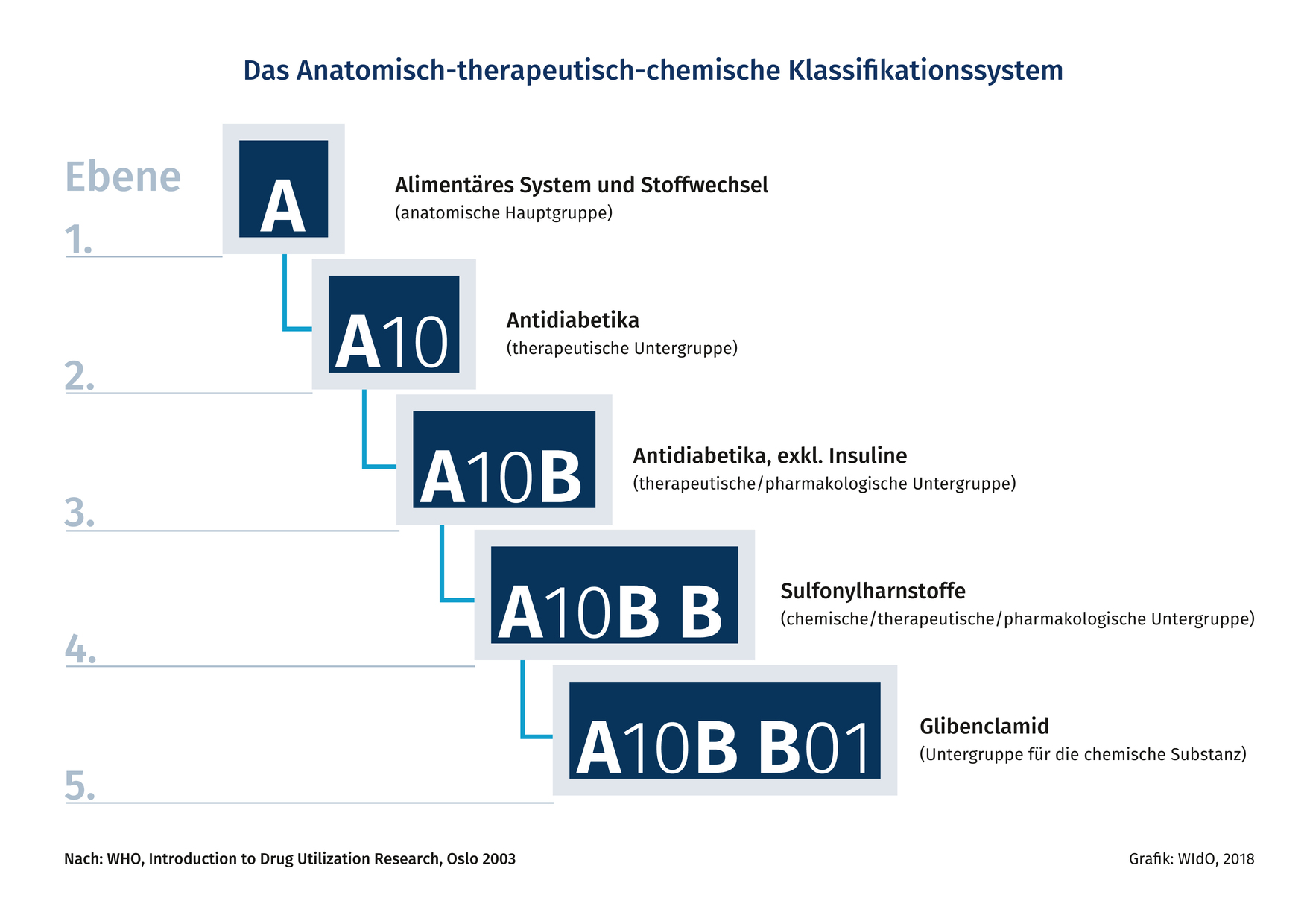
ATC
Anatomical Therapeutic Chemical is a classification system, the active substances are divided into different groups according to the organ or system on which they act and their therapeutic, pharmacological and chemical properties. Drugs are classified in groups at five different levels
ATC
Problem
Solution ?
Enter RAMSES

RAMSES
RAMSES
ATC code
ATC code
Dose
- Haloperidol 0.5mg
- Haloperidol 1mg/ml
Dose
Dose
drugsatc
- DuckDB
- R package
- ATC to DDD mapping
- DDD calculation
drugsatc
https://github.com/SAFEHR-data/drugsatc
From OMOP to DDD: https://razekmh.github.io/talks